Chemical synapse
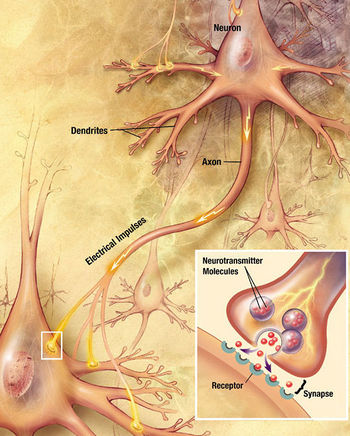
Chemical synapses are specialized junctions through which neurons signal to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.
At a chemical synapse, one neuron releases a neurotransmitter into a small space (the synapse) that is adjacent to another neuron. Neurotransmitters must then be cleared out of the synapse efficiently so that the synapse can be ready to function again as soon as possible.
The adult human brain is estimated to contain from 1014 to 5 × 1014 (100-500 trillion) synapses. Every cubic millimeter of cerebral cortex contains roughly a billion of them.[1]
The word "synapse" comes from "synaptein", which Sir Charles Scott Sherrington and colleagues coined from the Greek "syn-" ("together") and "haptein" ("to clasp"). Chemical synapses are not the only type of biological synapse: electrical and immunological synapses also exist. Without a qualifier, however, "synapse" commonly means chemical synapse.
Contents |
Structure
| Structure of a typical chemical synapse |
|---|
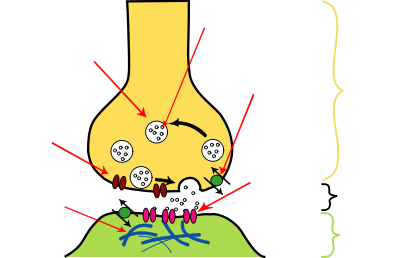
Postsynaptic
density Voltage-
gated Ca++ channel Synaptic
vesicle Reuptake
pump Receptor
Axon terminal
|
Synapses are functional connections between neurons, or between neurons and other types of cells.[2][3] A typical neuron gives rise to several thousand synapses, although there are some types that make far fewer.[4] Most synapses connect axons to dendrites,[5][6] but there are also other types of connections, including axon-to-cell-body[7][8], axon-to-axon,[7][8] and dendrite-to-dendrite.[6] Synapses are generally too small to be recognizable using a light microscope except as points where the membranes of two cells appear to touch, but their cellular elements can be visualized clearly using an electron microscope.
Chemical synapses pass information directionally from a presynaptic cell to a postsynaptic cell and are therefore asymmetric in structure and function. The presynaptic terminal, or synaptic bouton, is a specialized area within the axon of the presynaptic cell that contains neurotransmitters enclosed in small membrane-bound spheres called synaptic vesicles. Synaptic vesicles are docked at the presynaptic plasma membrane at regions called active zones (AZ).
Immediately opposite is a region of the postsynaptic cell containing neurotransmitter receptors; for synapses between two neurons the postsynaptic region may be found on the dendrites or cell body. Immediately behind the postsynaptic membrane is an elaborate complex of interlinked proteins called the postsynaptic density (PSD).
Proteins in the PSD are involved in anchoring and trafficking neurotransmitter receptors and modulating the activity of these receptors. The receptors and PSDs are often found in specialized protrusions from the main dendritic shaft called dendritic spines.
Between the pre- and postsynaptic cells is a gap about 20 nm wide called the synaptic cleft. The small volume of the cleft allows neurotransmitter concentration to be raised and lowered rapidly. The membranes of the two adjacent cells are held together by cell adhesion proteins.[9]
Signaling in chemical synapses
Overview
Here is a summary of the sequence of events that take place in synaptic transmission from a presynaptic neuron to a postsynaptic cell. Each step is explained in more detail below. Note that with the exception of the final step, the entire process may run only a few tenths of a millisecond, in the fastest synapses.
- The process begins with a wave of electrochemical excitation called an action potential traveling along the membrane of the presynaptic cell, until it reaches the synapse.
- The electrical depolarization of the membrane at the synapse causes channels to open that are permeable to calcium ions.
- Calcium ions flow through the presynaptic membrane, rapidly increasing the calcium concentration in the interior.
- The high calcium concentration activates a set of calcium-sensitive proteins attached to vesicles that contain a neurotransmitter chemical.
- These proteins change shape, causing the membranes of some "docked" vesicles to fuse with the membrane of the presynaptic cell, thereby opening the vesicles and dumping their neurotransmitter contents into the synaptic cleft, the narrow space between the membranes of the pre- and post-synaptic cells.
- The neurotransmitter diffuses within the cleft. Some of it escapes, but some of it binds to chemical receptor molecules located on the membrane of the postsynaptic cell.
- The binding of neurotransmitter causes the receptor molecule to be activated in some way. Several types of activation are possible, as described in more detail below. In any case, this is the key step by which the synaptic process affects the behavior of the postsynaptic cell.
- Due to thermal shaking, neurotransmitter molecules eventually break loose from the receptors and drift away.
- The neurotransmitter is either reabsorbed by the presynaptic cell, and then repackaged for future release, or else it is broken down metabolically.
Neurotransmitter release
The release of a neurotransmitter is triggered by the arrival of a nerve impulse (or action potential) and occurs through an unusually rapid process of cellular secretion, also known as exocytosis: Within the presynaptic nerve terminal, vesicles containing neurotransmitter sit "docked" and ready at the synaptic membrane. The arriving action potential produces an influx of calcium ions through voltage-dependent, calcium-selective ion channels at the down stroke of the action potential (tail current).[10] Calcium ions then bind with the proteins found within the membranes of the synaptic vesicles, allowing the vesicles to "dock" with the presynaptic membrane resulting in the creation of a fusion pore. The vesicles then release their contents to the synaptic cleft through this fusion pore[11] within 180µsec of calcium entry.[10] Vesicle fusion is driven by the action of a set of proteins in the presynaptic terminal known as SNAREs.
The membrane added by this fusion is later retrieved by endocytosis and recycled for the formation of fresh neurotransmitter-filled vesicles.
Receptor binding
Receptors on the opposite side of the synaptic gap bind neurotransmitter molecules and respond by opening nearby ion channels in the postsynaptic cell membrane, causing ions to rush in or out and changing the local transmembrane potential of the cell. The resulting change in voltage is called a postsynaptic potential. In general, the result is excitatory, in the case of depolarizing currents, or inhibitory in the case of hyperpolarizing currents. Whether a synapse is excitatory or inhibitory depends on what type(s) of ion channel conduct the postsynaptic current display(s), which in turn is a function of the type of receptors and neurotransmitter employed at the synapse.
Termination
After a neurotransmitter molecule binds to a receptor molecule, it must be removed to allow for the postsynaptic membrane to continue to relay subsequent EPSPs and/or IPSPs. This removal can happen through one or more processes:
- The neurotransitter may diffuse away due to thermally-induced oscillations of both it and the receptor, making it available to be broken down metabolically outside the neuron or to be reabsorbed.[12]
- Enzymes within the subsynaptic membrane may inactivate/metabolize the neurotransmitter.
- Reuptake pumps may actively pump the neurotransmitter back into the postsynaptic axon terminal for reprocessing and re-release following a later action potential.[12]
The time frame for these "clearing" processes varies greatly for different types of synapses, ranging from a few tenths of a millisecond for the fastest, to several seconds for the slowest.
Modulation of synaptic transmission
Synaptic transmission can be modulated by e.g. desensitization, homosynaptic plasticity and heterosynaptic plasticity:
Desensitization
Desensitization of the postsynaptic receptors is a decrease in response to the same neurotransmitter stimulus. It means that the strength of a synapse may in effect diminish as a train of action potentials arrive in rapid succession--a phenomenon that gives rise to the so-called frequency dependence of synapses. The nervous system exploits this property for computational purposes, and can tune its synapses through such means as phosphorylation of the proteins involved.
Homosynaptic plasticity
Homosynaptic plasticity (or also homotropic modulation) is a change in the synaptic strength that results from the history of activity at a particular synapse. This can result from changes in presynaptic calcium as well as feedback onto presynaptic receptors, i.e. a form of autocrine signaling. Homosynaptic plasticity can affect the number and replenishment rate of vesicles or it can affect the relationship between calcium and vesicle release. Homosynaptic plasticity can also be post-synaptic in nature. It can result in either an increase or decrease in synaptic strength.
One example is neurons of the sympathetic nervous system (SNS), which release noradrenaline, which, besides affecting postsynaptic receptors, also affects presynaptic α2-adrenergic receptors, inhibiting further release of noradrenaline. [13] This effect is utilized with clonidine to perform inhibitory effects on the SNS.
Heterosynaptic plasticity
Heterosynaptic plasticity (or also heterotropic modulation) is a change in synaptic strength that results from the activity of other neurons. Again, the plasticity can alter the number of vesicles or their replenishment rate or the relationship between calcium and vesicle release. Additionally, it could directly affect calcium influx. Heterosynaptic plasticity can also be post-synaptic in nature, affecting receptor sensitivity.
One example is again neurons of the sympathetic nervous system, which release noradrenaline, which, in addition, generate inhibitory effect on presynaptic terminals of neurons of the parasympathetic nervous system.[13]
Effects of drugs
One of the most important features of chemical synapses is that they are the site of action for the majority of psychoactive drugs. Synapses are affected by drugs such as curare, strychnine, cocaine, morphine, alcohol, LSD, and countless others. These drugs have different effects on synaptic function, and often are restricted to synapses that use a specific neurotransmitter. For example, curare is a poison which stops acetylcholine from depolarising the post-synaptic membrane, causing paralysis. Strychnine blocks the inhibitory effects of the neurotransmitter glycine, which causes the body to pick up and react to weaker and previously ignored stimuli, resulting in uncontrollable muscle spasms. Morphine acts on synapses that use endorphin neurotransmitters, and alcohol increases the inhibitory effects of the neurotransmitter GABA. LSD interferes with synapses that use the neurotransmitter serotonin. Cocaine blocks reuptake of dopamine and therefore increases its effects.
Integration of synaptic inputs
In general, if an excitatory synapse is strong, an action potential in the presynaptic neuron will trigger another in the postsynaptic cell, whereas, at a weak synapse, the excitatory postsynaptic potential ("EPSP") will not reach the threshold for action potential initiation. In the brain, however, each neuron forms synapses with many others, and, likewise, each receives synaptic inputs from many others. When action potentials fire simultaneously in several neurons that weakly synapse on a single cell, they may initiate an impulse in that cell even though the synapses are weak. This process is known as summation.[14] On the other hand, a presynaptic neuron releasing an inhibitory neurotransmitter such as GABA can cause inhibitory postsynaptic potential in the postsynaptic neuron, decreasing its excitability and therefore decreasing the neuron's likelihood of firing an action potential. In this way, the output of a neuron may depend on the input of many others, each of which may have a different degree of influence, depending on the strength of its synapse with that neuron. John Carew Eccles performed some of the important early experiments on synaptic integration, for which he received the Nobel Prize for Physiology or Medicine in 1963. Complex input/output relationships form the basis of transistor-based computations in computers, and are thought to figure similarly in neural circuits.
Synaptic strength
The strength of a synapse is defined by the change in transmembrane potential resulting from activation of the postsynaptic neurotransmitter receptors. This change in voltage is known as a postsynaptic potential, and is a direct result of ionic currents flowing through the postsynaptic ion channels. Changes in synaptic strength can be short–term and without permanent structural changes in the neurons themselves, lasting seconds to minutes — or long-term (long-term potentiation, or LTP), in which repeated or continuous synaptic activation can result in second messenger molecules initiating protein synthesis, resulting in alteration of the structure of the synapse itself. Learning and memory are believed to result from long-term changes in synaptic strength, via a mechanism known as synaptic plasticity.
Volume transmission
When a neurotransmitter is released at a synapse, it reaches its highest concentration inside the narrow space of the synaptic cleft, but some of it is certain to diffuse away before being reabsorbed or broken down. If it diffuses away, it has the potential to activate receptors that are located either at other synapses or on the membrane away from any synapse. The extrasynaptic activity of a neurotransmitter is known as volume transmission.[15] It is well established that such effects occur to some degree, but their functional importance has long been a matter of controversy.[16]
Recent work indicates that volume transmission may be the predominant mode of interaction for some special types of neurons. In the mammalian cerebral cortex, a class of neurons called neurogliaform cells can inhibit other nearby cortical neurons by releasing the neurotransmitter GABA into the extracellular space. Approximately 78% of neurogliaforms do not form classical synapses. This may be the first definitive example of neurons communicating chemically where synapses are not present.[17]
Relationship to electrical synapses
An electrical synapse is a mechanical and electrically conductive link between two abutting neurons that is formed at a narrow gap between the pre- and postsynaptic cells known as a gap junction. At gap junctions, cells approach within about 3.5 nm of each other, rather than the 20 to 40 nm distance that separates cells at chemical synapses.[18][19] As opposed to chemical synapses, the postsynaptic potential in electrical synapses is not caused by the opening of ion channels by chemical transmitters, but by direct electrical coupling between both neurons. Electrical synapses are therefore faster[9] and more reliable than chemical synapses. Electrical synapses are found throughout the nervous system, yet are less common than chemical synapses.
See also
- Neuroscience
- Postsynaptic potential
-
- Excitatory postsynaptic potential
- Inhibitory postsynaptic potential
- Immunological synapse
- Neuromuscular junction
- Neurotransmitter
- Receptor
- Exocytosis
- Ribbon synapse
Notes
- ↑ Alonso-Nanclares L, Gonzalez-Soriano J, Rodriguez JR, DeFelipe J. (2008). Gender differences in human cortical synaptic density. Proc Natl Acad Sci U S A. 105(38):14615-9.PubMed
- ↑ Rapport, Richard L. (2005) (Digitised online by Googlebooks). Nerve Endings: The Discovery of the Synapse. W. W. Norton & Company. pp. 1–37. ISBN 0393060195, 9780393060195. http://books.google.com/books?id=fWBBIUhoLzMC&printsec=frontcover&dq=Synapse&lr=&as_brr=3&as_pt=ALLTYPES. Retrieved 2008-12-26.
- ↑ Squire, Larry R.; Floyd Bloom, Nicholas Spitzer (2008) (Digitised online by Googlebooks). Fundamental Neuroscience. Academic Press. pp. 425–426. ISBN 0123740193, 9780123740199. http://books.google.com/books?id=GOxrtYzmixcC. Retrieved 2008-12-26.
- ↑ Hyman, Steven E.; Eric Jonathan Nestler (1993) (Digitised online by Googlebooks). The Molecular Foundations of Psychiatry. American Psychiatric Pub. pp. 425–426. ISBN 0880483539, 9780880483537. http://books.google.com/books?id=pI4ayEWvcQkC&pg=PA24&dq=neuron+gives+rise+to+several+thousand+synapses&lr=&as_brr=3&as_pt=ALLTYPES. Retrieved 2008-12-26.
- ↑ Smilkstein, Rita (2003). We're Born to Learn: Using the Brain's Natural Learning Process to Create Today's Curriculum. Corwin Press. p. 56. ISBN 076194642X, 9780761946427. http://books.google.com/books?id=6ZHELyI9XEIC&pg=PA56&dq=synapses+connect+axons+to+dendrites&lr=&as_brr=3&as_pt=ALLTYPES. Retrieved 2008-12-26.
- ↑ 6.0 6.1 Lytton, William W. (2002). From Computer to Brain: Foundations of Computational Neuroscience. Springer. p. 28. ISBN 0387955267, 9780387955261. http://books.google.com/books?id=vfL4uxiWZ9YC&pg=PA28&dq=synapses+connect+dendrites+to+dendrites&lr=&as_brr=3&as_pt=ALLTYPES. Retrieved 2008-12-26. Axons connecting dendrite to dendrite are dendrodendritic synapses. Axons which connect axon to dendrite are called axodendritic synapses
- ↑ 7.0 7.1 Garber, Steven D. (2002). Biology: A Self-Teaching Guide. John Wiley and Sons. p. 175. ISBN 0471223301, 9780471223306. http://books.google.com/books?id=h-rCfjJGFb4C&pg=PA175&dq=synapses+connect+axons+to+cell+body&lr=&as_brr=3&as_pt=ALLTYPES. Retrieved 2008-12-26.
- ↑ 8.0 8.1 Weiss, Mirin; Dr Steven M. Mirin, Dr Roxanne Bartel (1994). Cocaine. American Psychiatric Pub. p. 52. ISBN 1585621382, 9781585621385. http://books.google.com/books?id=efqxJ5jhOaEC&pg=PA52&dq=synapses+connect+axons+to+cell+body&lr=&as_brr=3&as_pt=ALLTYPES. Retrieved 2008-12-26. Axons terminating on the postsynaptic cell body are axosomatic synapses. Axons terminate on axons are axoaxonic syanpses
- ↑ 9.0 9.1 Kandel et al., 2000, p. 182
- ↑ 10.0 10.1 Llinás R, Steinberg IZ, Walton K (1981). "Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse". Biophysical Journal 33 (3): 323–351. PMID 6261850. http://www.biophysj.org/cgi/pmidlookup?view=long&pmid=6261850.
- ↑ (Carlson, 2007, p.56)
- ↑ 12.0 12.1 Sherwood L.,stikawy (2007). Human Physiology 6e: From Cells to Systems
- ↑ 13.0 13.1 Pharmacology, (Rang, Dale, Ritter & Moore, ISBN 0-443-07145-4, 5:th ed., Churchill Livingstone 2003) Page 129
- ↑ Single Neurons Are Complex Computation Devices in Chapter 11 of "Molecular Biology of the Cell, 4th Ed." by Bruce Alberts, et al. (2001) Garland Science Textbooks, ISBN 0-8153-3218-1.
- ↑ Zoli M, Torri C, Ferrari R, et al. (1998). "The emergence of the volume transmission concept". Brain Res. Brain Res. Rev. 26: 136–47. PMID 9651506.
- ↑ Fuxe K, Dahlström A, Höistad M, et al. (2007). "From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission". Brain Res Rev 55: 17–54. doi:10.1016/j.brainresrev.2007.02.009. PMID 17433836.
- ↑ Oláh S, Füle M, Komlósi G, et al. (2009). "Regulation of cortical microcircuits by unitary GABA-mediated volume transmission". Nature 461: 1278–81. doi:10.1038/nature08503. PMID 19865171.
- ↑ Kandel et al., 2000, p. 176
- ↑ Hormuzdi et al., 2004
References
- Carlson, Neil R. (2007). Physiology of Behavior (9th edition ed.). Boston, MA: Pearson Education, Inc. ISBN 0205593895.
- Kandel, Eric R.; James H. Schwartz, Thomas M. Jessell (2000). Principles of Neural Science (4th edition ed.). New York: McGraw-Hill. ISBN 0-8385-7701-6.
- Llinas R. Sugimori M. and Simon S.M. (1982) PNAS 79:2415-2419
- Llinás R, Steinberg IZ, and Walton K (1981). Biophysical Journal 33: 323–352.
- Bear, Mark; Mark F. Bear, Barry W. Connors, Michael A. Paradiso (2001). Neuroscience: Exploring the Brain. Hagerstown, MD: Lippincott Williams & Wilkins. ISBN 0-7817-3944-6.
- Hormuzdi, SG; Filippov MA, Mitropoulou G, Monyer H, Bruzzone R. (March 2004). "Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks". Biochim Biophys Acta 1662 (1-2): 113–137. doi:10.1016/j.bbamem.2003.10.023. PMID 15033583.
- Karp, Gerald (2005). Cell and Molecular Biology: concepts and experiments (4th ed.). Hoboken, NJ: John Wiley & Sons, Inc. ISBN 0-471-46580-1..
- Nicholls, J.G.; Martin A.R., Wallace B.G., Fuchs P.A. (2001). From Neuron to Brain (4th ed.). Sunderland, MA: Sinauer Associates. ISBN 0878934391.
External links
- Synapse Review for Kids
- Synapses Biologymad.com (2004)
- Synapse – Cell Centered Database
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||